
Let me start this article by saying that algaecides should never be used alone. It may seem like such a simple situation: you see algae, you spray chemicals, algae dies, and the lake goes back to normal. But like everything else in life, things are never that simple.
Let's start with the basics. Algae growth occurs when there is an excess of nutrients in the lake. It is encouraged by warm temperature and available sunlight. When algae starts to spread rapidly, that's when you have a problem. Not only will your water look and smell disgusting, but that algae will start sucking nutrients from the water, which starves everything else in the lake, eventually including the algae itself.
At this point it's time to use an algaecide. Which type of algaecide should you use? Well, in keeping with our theme of complexity, we'll have to go with: it depends. There are many different types of algaecides and herbicides with different active ingredients (and different formulations of those active ingredients, but we'll get to that later). There are contact algaecides such as Endothall, Diquat, and Copper Sulfate, and systemic materials like Glyphosate and Fluridone. The main difference between the two is that contact algaecides are fast acting and localized while systemic algaecides are absorbed into the aquatic life in order to kill it, so they generally take a longer time and need to spread throughout the water body. Depending on the type of problem algae, certain chemicals will not be as effective as others (and other chemicals will do nothing at all).
Not all chemicals are created equal. Or rather, not all formulations of chemicals are created equal. If we had the time (and 500 sheets of paper) we could go over each active ingredient found in common algaecides and herbicides, and analyze where they are most effective, but for now we'll just stick with copper sulfate products. Why? Well, honestly because I know the most about them. But also they are one of the most effective and widely used type of algaecide.
Copper Sulfate has been an industry standard for algae control in the U.S. since 1905. Algae treatment started with the Roman Empire, lining public baths with copper to help disinfect the waters. Since then copper usage has progressed, and has been further developed for the field of algae treatment.

How it works:
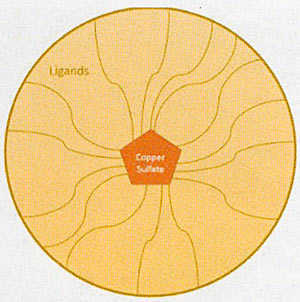
Copper sulfate is a poison. When it comes into contact with the algae it kills it, which is what you want after all. It works on most types of algae, which is why it is very commonly used. Copper sulfate itself is a granular substance mined from the earth but, it in this form, it does not have a long contact time. Meaning? It will kill the algae it comes into contact with, but once it falls through the water column it stays in the sediments and does practically nothing.
How did they fix this problem? It's a process called chelation. Chelation basically means surrounding the copper sulfate with other molecules (called ligands), which prevents the copper sulfate from falling through the water column so quickly. Imagine the candy coating on an M&M. It's protecting the chocolate inside from melting in your hand until it's time to melt in your mouth. Same idea with chelation—it protects the copper ion from reacting too quickly, so the overall treatment time is extended and more algae is killed with less copper. Now, to make things more complicated, there are multiple types of ligands that can be used during chelation. Does it actually make a difference? Yes. Yes it does.

There are two main types of ligands; synthetic and organic. Synthetic ligands tend to hold onto the copper sulfate a little too tightly. This means that the synthetic chelated copper will stay in the water for longer (imagine a thicker candy coating). While this may initially appear beneficial, it hampers the ionic copper activity, which is what is actually killing the algae. Usually, manufacturers compensate by adding more copper. This doesn't sound too bad, but you want to minimize the copper in the water because that will minimize the copper that ends up in your sediments. Too much buildup of copper in the sediment will cause alot bigger problems later on.
The organic ligands seem to be a good balance between holding on to the copper sulfate too tightly, and not tightly enough. This allows for a significantly lower copper concentration with the same kill rate as the other copper sulfate products.
I'm going to continue this article by saying algaecides should never be used alone. Period.
No matter which ones you use. Chelated or not. Never. Why not? The algae is dead now, no more issues, right? Well you've just killed all of your algae and now their little algae carcasses are sinking in the water. All of a sudden the giant mass of green that was taking all of the nutrients from the water has now turned into a giant mass of nutrients for bacteria to eat. Sometimes the bacteria can keep up with this inflow of nutrients. They can break it down quickly and the lake will return to a normal state. But when the lake's ecosystem has been weakened (say from a massive bloom of algae killing off the bacteria) the bacteria may not be able to decompose the dead algae fast enough. The water will have an excess of nutrients. Throw in some warm weather and lots of sunlight, and you've got yourself another algae bloom.
So what can you do about it? Four simple words. Kill. Chop. Drop. Eat. You've already gotten the kill out of the way. So let's move on.
Chop:
Now that the algae is just floating around, you need to help out your bacteria. The first step is to add enzymes. Enzymes basically speed up different reactions in the decomposition process. They are cutting the algae into little pieces (metaphorically) to make it easier for the bacteria to eat them.
Drop:
Sometimes you'll run into a problem where the algae particles are so small that they never really settle out of the water, they just remain suspended throughout the water column. This will definitely not be a pretty sight, and will make it more difficult for bacteria to find the algae. So you use a flocculent. This will help the tiny particles bind together so that they will be heavy enough to fall to the bottom of the lake. That is where the real action starts.
Eat:
Bacteria that lives on the lake bed (benthic bacteria) are the real heroes here. They are the main decomposers of the ecosystem. You want lots of them, you want them healthy, and you want them working on your side. Sometimes it may be a good idea to add new bacteria into the system. There are plenty of beneficial bacteria products that will work wonders for your decomposing. Once this bacteria has worked their magic with the dead algae THEN your lake is back to normal.
Does this mean that you can stop all maintenance and care? Of course not. Prevention is always important, and keeping a water system healthy will always give you plenty of additional benefits. The most important thing to remember here is to never just toss an algaecide into your lake. You need to treat your lake like an entire ecosystem, because—well, it is one!
Reprinted with permission from Pond Boss Magazine


