Water is two parts hydrogen and one part oxygen (H20), but hydrogen is much lighter than oxygen, and water is, by weight, 88.89 percent oxygen. However, oxygen in the water molecules is unavailable for animals to use for respiration. Fish stress and mortality can result from low dissolved oxygen concentration in fish ponds. Land animals breathe atmospheric air into their lungs and absorb molecular oxygen (02). Fish have gills through which they absorb molecular oxygen that has dissolved in water from the atmosphere or been released into the water by photosynthesis of aquatic plants. We rarely hear of land animals suffocating, but suffocation (lack of molecular oxygen) is relatively common in fish.
The air near the earth's surface contains 20.95 percent molecular oxygen. The amount of molecular oxygen that will dissolve in water varies with temperature and atmospheric pressure. At standard atmospheric pressure (29.92 inches of mercury), the solubility of oxygen in water ranges from 12.76 parts per million (ppm) in liquid water at 5°C (41°F) to 6.94 ppm at 35°C or 95°F (Table 1).
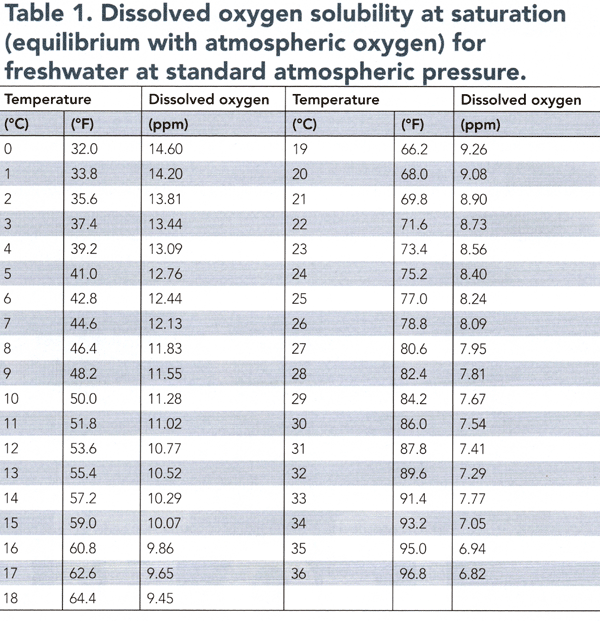
Pond water usually has a somewhat higher dissolved oxygen concentration than its saturation level (Table 1) for molecular oxygen during daytime and less than the saturation level during nighttime. These changes result because the release of oxygen by aquatic plants during the day usually exceeds the combined loss of dissolved oxygen through the respiration of the pond biota and the net diffusion of dissolved oxygen into the air. At night, photosynthesis stops, but respiration usually continues at a rate more significant than the rate at which atmospheric oxygen can diffuse into the water.
The alveoli in the lungs of a land animal can be exposed to 1 milligram of molecular oxygen by exposure to about 4.8 milligrams of air (1 mg 02 4 0.2095 mg 02/mg air). Water at 25°C (77°F) contains 8.24 milligrams per liter (same as ppm) of molecular oxygen. Exposing fish's gills to 1 milligram molecular oxygen requires 0.121 L of water (1 mg 02 4 8.24 mg 02/L) weighing 118,200 milligrams. A fish must pump a volume of water weighing about 25,000 times more than the air that a land animal must breathe to expose respiratory surfaces to the same amount of molecular oxygen.
The energy for pumping water across the gills will increase as the dissolved oxygen concentration decreases. For example, at 3 ppm dissolved oxygen, 0.333 liters weighing 332,000 milligrams would have to be pumped to provide 1 milligram of dissolved oxygen across the gill surface—2.8 times more than when water at 25°C is saturated with dissolved oxygen.
Government standards typically allow around four people per square yard to stand and view outdoor events. This equates to around 19,000 people/acre. At an average weight of 150 pounds/person, this would equal about 2,900,000 pounds of people per acre. A 1-acre pond of 6 feet average depth would contain 134 pounds of dissolved oxygen at 25°C. A fish biomass of 2,900,000 pounds would deplete the dissolved oxygen within about 4.5 minutes, and the fish would all die. On the other hand, an equivalent biomass of people at an outdoor event would be crowded but doing fine and hopefully enjoying themselves after several hours.
When land animals use oxygen from the air, oxygen in the air is rapidly replaced by air circulation around them. When fish remove oxygen from water, the replacement rate of water around them with water of greater dissolved oxygen concentration is slower than the replacement rate for air around land animals. Air circulates more freely than water because it is about 900 times lighter than water. When dissolved oxygen is removed from the water at night, it must be replaced by diffusion of atmospheric oxygen into the water, and water circulation must move the dissolved oxygen from the surface film into the water column where the fish usually hang out. Because the surface film of water is always nearly saturated with dissolved oxygen, fish come to the surface and gasp to use this source of oxygen when the pond water column is depleted of dissolved oxygen.
The discussion above shows why dissolved oxygen is critical in sportfish ponds. In fact, except for ponds that need liming, water quality problems in sportfish ponds with adequate dissolved oxygen concentrations should be few. Low dissolved oxygen concentration not only has direct adverse effects on fish, but it can also result in elevated concentrations of potentially toxic metabolites, especially ammonia and nitrite, and it can exacerbate other water quality issues.
The primary safeguard against low dissolved oxygen in sportfish ponds is to avoid excessive fertilizer applications, which lead to overly dense phytoplankton blooms that lead to low dissolved oxygen concentrations at night. Thick blooms also encourage shallow thermal stratification, which can cause dissolved oxygen depletion in the event of sudden destratification following heavy rains or winds in summer. Moreover, dense blue-green algae blooms may suddenly die, and the decay of dead algae can cause oxygen depletion.
Thermal stratification in ponds can be avoided by using diffuser aerators, which release air in deep water areas to mix the water column. Surface aerators may also be used for low dissolved oxygen concentration, but sportfish pond owners seldom have aerators.
Dr. Claude Boyd is a professor emeritus in the School of Fisheries, Aquaculture and Aquatic Sciences, Auburn University, Auburn, Alabama 36849. His work with water quality is internationally renowned. His most recent book, Handbook for Aquaculture Water Quality, is a must for anyone interested in learning about water chemistry and how it relates to your pond. It's technical thorough, but easy to read and understand. Buy it at www.pondboss.com in the online store.



