One of the easiest pond water quality variables to measure is pH, because inexpensive devices for determining pH are available to pond owners. Aside from water temperature, pH is probably the most commonly measured water quality variable. The pH also is a misunderstood variable, and the results of its measurement often confuses pond owners.
The chemist's definition of pH is the negative logarithm of the hydrogen ion activity. Although this definition may not be very informative to many pond owners, most do recognize the pH scale and understand pH below 7 to mean acidic and pH above 7 to mean alkaline (or basic).
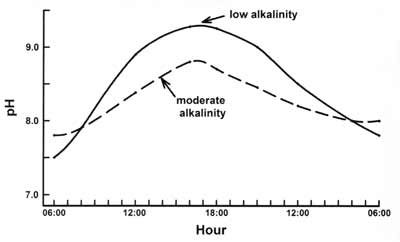
Moderation is usually a good position in life and in nature, and water pH in fish ponds is no exception. A pH range of 6.0-8.5 is acceptable in fish ponds (Table 1) and pH 7-8 is likely ideal. However, the pH fluctuates in a fish pond (Fig. 1); surface water pH might be 6.5 at dawn but reach 9.5 by midafternoon in a low alkalinity water. In a water with moderate alkalinity concentration (30-150 mg/L), surface water pH may be 7.5 in the morning and only reach 8.5-9.0 in the afternoon. Pond owners typically measure pH of surface water, but pH declines with greater water depth. Also, if the pH is considered over a period of time, the daily fluctuation usually is greater on clear days than on cloudy days, and it also fluctuates more when there is more phytoplankton than when there is less phytoplankton.
In ponds with alkalinity above 150 mg/L, the morning pH may be 8 or even above. However, afternoon pH usually will not rise above 9 or 9.5 in surface water.
The ambient pH of pond water depends mainly on the nature of pond bottom soils and watershed soils. In areas with acidic soils, natural water will have low alkalinity and relatively low pH. Ponds with low alkalinity water and located in wooded areas, and low alkalinity ponds with dense stands of marginal and emergent aquatic plants also tend to be stained with humic substances. Phytoplankton does not grow well in such ponds because of low nutrient availability, restricted light penetration, or both. When treated with nitrogen and phosphorus fertilizers, such waters usually develop phytoplankton blooms and there will be wide daily swings in pH.
The simple explanation for these pH fluctuations is that carbon dioxide is acidic in water. Phytoplankton removes carbon dioxide for use in photosynthesis causing daytime pH to increase. There is a limited amount of carbon dioxide in low alkalinity water, and when pH reaches 8.3, carbon dioxide depletion occurs. Aquatic plants extract carbon dioxide from bicarbonate, and photosynthesis continues at pH above 8.3. But, extraction of C02 from bicarbonate results in the release of carbonate into the water. Carbonate hydrolyzes to cause pH to continue to climb, and in low alkalinity waters, pH may reach 9-10. At night, photosynthesis stops for lack of light but the continuing release of carbon dioxide into the water by respiration results in a decrease in pH.
The solution to the high pH problem in low alkalinity waters mentioned above is to apply liming materials to ponds to increase the alkalinity. This increases the availability of carbon dioxide and lessens the pH fluctuation between day and night. The process of minimizing pH change is known as buffering, and alkalinity is the buffer in pond waters.

If ponds are limed so that alkalinity is 30-40 mg/L or more, extreme pH fluctuations resulting from low alkalinity and poor buffering capacity can be avoided. The pH fluctuations that occur in surface water of properly managed sportfish ponds may still be as much as two units, e.g., 6.5- 9.0 or 7-9.5. The pH declines with greater water depth and fish do stay at the surface; they can occupy any depth in a pond where there is adequate dissolved oxygen. The pH at 3 to 5 feet below the surface may be 1 unit or more less than at the surface.
The take away from the discussion above is to lime acidic ponds to increase alkalinity to 30 mg/L or more and buffer water against pH change. The pH will still change, because it is normal in water bodies for pH to increase in daytime and decrease at night, a result of changes in carbon dioxide concentration caused by differences in photosynthesis and respiration rates during a 24-hour period. However, the buffering provided by alkalinity will protect against excessively high or low pH.
In ponds with high alkalinity, the pH may be above 8-8.5 most of the time, but afternoon pH will usually be no greater than in ponds of lower alkalinity. There are some arid areas where waters are so alkaline that high pH is detrimental to fish production. Unfortunately, there is no practical means of reducing the alkalinity of the typical sportfish pond.
As a general rule, measuring pond water pH frequently is a counterproductive activity that raises unwarranted concerns. But, if one is insistent on measuring pH, they should be aware of the fluctuations that occur naturally. The alkalinity is a more useful index of the suitability of water for fish culture.
Dr. Claude Boyd is a renowned aquaculture water chemistry expert and Professor Emeritus with the School of Fisheries, Aquaculture and Aquatic Sciences, Auburn University, Auburn, Alabama 36849. Dr. Boyd's latest book, Handbook for Aquaculture Water Quality, is available in the Pond Boss online store, or you can order one by calling Pond Boss, (800)687-6075 or (903)564-6144.
Reprinted with permission from Pond Boss Magazine



