
Few incidents are more sickening for a passionate pondmeister than strolling to that favorite water hole and seeing the shore lined with scaly creatures, upside down, floating everywhere. Fish kill. It's not the fish's fault. A huge majority of fish kills are related to deteriorating water quality. Managing water quality can be challenging. Too much aquatic vegetation, algae blooms, fish kills and foul odors are common pond ailments. Those are the effects. Causes may be an array of contributing factors which makes finding a solution that much more difficult. The best way to a workable solution is better understanding of components which impact the delicate balance of a pond's ecosystem. The most significant factors are: light and temperature, nutrients, and oxygen.
Light & Temperature
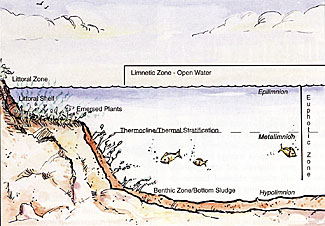
Sunlight is a lake's primary source of energy. Most energy which controls metabolism of a lake comes directly from solar energy utilized in photosynthesis. Photosynthesis occurs in the upper layer of a pond, called the euphotic zone. This is the area in the water where sunlight is able to penetrate. Shallow water, less than 9ft/3m deep, commonly experiences problem amounts of bottom-rooted weeds or benthic algae, because of sunlight penetration.
Thermal Stratification is a term meaning "temperature layering." As the spring and summer sun shines on a pond it warms surface water. Warm water expands, becoming lighter than those cooler, more dense waters trapped at pond's bottom. As the hot summer season progresses, the difference in temperature between warm surface waters and colder bottom waters increases. As a result, water stratifies or separates into layers. These layers do not mix all summer. The area created between warm and cold layers is called the thermocline or metalimnion. It is a physical barrier preventing vertical mixing.

Thermal stratification impacts water quality primarily because of its effect on dissolved oxygen levels. Compared to cooler water, warm water has a diminished capacity to hold oxygen. In fact, water at 52 Fahrenheit (11 Celsius) can hold 40% more oxygen than water at 80 Fahrenheit (27 Celsius.) As temperature increases, the water's capacity to hold oxygen decreases. Dissolved oxygen in a lake comes primarily from photosynthesis and wave/wind action. Warm upper layers have more oxygen than cold bottom waters because of photosynthesis and contact with the atmosphere. Bottom water is isolated. Therefore, bottom waters are typically anoxic. Since aquatic organisms require oxygen to survive, they must move from the anoxic area or die. Consequently, anoxic bottom waters lose zooplankton and aerobic bacteria necessary for efficient and effective digestion, while less effective, more pollutant tolerant forms of anaerobic bacteria develop.
The lack of dissolved oxygen sets in motion a series of chemical reactions that further degrade water quality: sulfide is converted to hydrogen sulfide, insoluble iron is converted to soluble forms, suspended solids increase and a severe decrease in the decomposition of waste materials on the pond bottom will occur. These natural chemical changes have a serious impact on water quality. Have you ever opened the drain of a pond, and caught a whiff of the first water out'? Nasty.
Shallow lakes offer the water manager an even greater challenge. Shallow ponds, less than 6ft/2m, tend to be warm enough for the entire water column to be productive with weed and algae growth. These types of lakes need extra consideration when determining the correct water management solution.
Nutrients
The second essential factor is nutrient impact. There is a direct correlation between available nutrients and populations of algae and aquatic weeds.
Where nutrients come from, how they are absorbed, broken down and their impact on water quality give valuable clues how to manage a pond. Diagnosis of a lake's chemical make up can help you design a preventative program for a problem lake.
Consider how organic nutrients accumulate and digest in a lake. Organic nutrients are carbon-based compounds essential to life of plants. In lake ecology, macro-nutrients are phosphorus and nitrogen. In fact, phosphorus has been identified as the single greatest contributor to aquatic plant growth; one grain of phosphorous can produce one hundred grams of algal biomass. As nutrient levels in water increase so do aquatic plant and weed growth. While some amounts of native species of plants are healthy for pond ecology, too much can lead to severe problems.
The three most common sources of nutrients are bottom silt and dead vegetation in the lake, runoff water from surrounding turf areas, and the sources of incoming water.
Bottom Silt and Vegetation in the Lake
Aquatic plants and sediment are primary nutrient sources. With a two-week life cycle, blue-green algae can experience cell division and double their population as often as every 20 minutes. At the end of the cycle, plants simply die and begin to sink, adding to biomass. The "aquatic compost pile" at the bottom, or benthic zone, deepens. The layer of dead plant material can feed future algae and aquatic weed blooms, a phenomenon called nutrient cycling. Nutrient cycling creates additional demands on available oxygen in the bottom waters, or hypolimmon.
Studies at the University of Florida indicate sediment, or sludge build up, can accumulate at a rate of 1 to 5 inches (2.5 to 12 cm) per year in temperate climates. In tropical climates the rate increases to 3 to 8 inches (6 to 16 cm) per year depending on levels of nutrient loading.
At a mid-point accumulation rate of 3 inches (7cm) per year, one surface acre (4000 ml) lake will lose 80,000 gallons of water storage capacity in a single year. Imagine the impact on an irrigation storage basin over the course of ten, twenty or fifty years. Sludge build up can gradually occur, robbing any lake or irrigation basin of its capacity to store water.
Run Off
The second common source of nutrients is runoff from surrounding turf, roads, farms and other outlying areas. The USGA reports up to 4% of fertilizers applied to areas adjacent to ponds and lakes may eventually run off, known as nutrient loading. Consider that a golf course may apply up to sixteen tons of fertilizer in a year the possibility for a half ton of fertilizer to run off into lakes or drainage basins exists. Leaves, grass clippings, and other materials also run off, placing additional burdens on water's natural cleansing processes. Ponds and lakes often act as Mother Nature's "garbage cans." As nutrient levels increase, the rate of plant growth increases.
A case study presented by the North American Lake Management Society (NALMS) suggests algae can absorb over 1 mg\L of phosphorus and over 2.5mg\L of nitrogen. Nutrients have a significant impact on algae and aquatic weed growth; increased nutrient levels usually mean increased plant levels.
Incoming Water Sources
Nutrients are also added to lakes and ponds through inlet waters from effluent, wastewater treatment plants and leeching from septic systems. Inlet waters often have minimal oxygen and are loaded with phosphorus.
Oxygen
The third essential factor in lake and pond ecology is oxygen. Oxygen is crucial to all forms of life in the lake, and supports the food chain. A healthy ecosystem in a lake contains a wide variety of plants and animals including a natural mechanism to biodegrade organic nutrients. The bottom of the food chain consists of microscopic algae which are consumed by slightly larger zooplankton. Each level of consumer transfers a small fraction of energy the lake receives up the food chain. This means a few sport fish depend on a much larger supply of smaller fish, and in turn smaller fish depend on a large base of plants and algae, and large masses of plants and algae require an even larger amount of nutrients. As you can see a healthy food chain can pull a tremendous amount of nutrients out of the water, and oxygen supports this entire system.
Natural decomposition processes in the aquatic ecosystem are oxygen dependent. Aerobic digestion is a fast and efficient way of breaking down organic matter into fundamental nutrients. Moreover, an abundant supply of dissolved oxygen supports oxidation and other chemical processes which help keep the lake in ecological balance.
Aquatic plants and algae produce large amounts of oxygen through the process of photosynthesis. This is an important source of oxygen in most lakes especially older, or eutrophic lakes. At night, plants become oxygen consumers in the process of respiration, the opposite of photosynthesis. The surface area of a lake is increased by surface waves or ripples caused by wind, exposing more surface area the atmospheric oxygen. Surface water absorbs oxygen similar to a sponge.
When oxygen levels drop below 3 to 4 parts per million (PPM) oxygen stress occurs. Most fish begin to suffocate at such low oxygen concentrations.
Typical situations when oxygen stress happens are:
- Late at night and just before dawn
- Cloudy and still days
- Hot and humid days
- When lake's dissolved nutrient content is high
- After a chemical application
Most of these conditions are related directly to the amount of greenery or living biomass in a pond. Immediate reactions to oxygen depletion would be fish kills or pungent, musty or `rotten egg' odors. Long term issues include nutrient build up, sludge accumulation, and a chemical imbalance in the lake.
Nature has a clean up process that metabolizes or decomposes excess nutrients called organic digestion. Two types of naturally occurring bacteria are present in all lakes and ponds, aerobic and anaerobic. Bacteria work to break down nutrient loads by feeding on organic matter and digesting it into non-organic compounds which algae and aquatic plants can not readily use for food.
The most effective are aerobic bacteria. Aerobic bacteria only live in the presence of oxygen and they metabolize or break down nutrients while respiring or consuming oxygen in the process. They are efficient, roughly seven times faster in organic digestion than anaerobic bacteria.
Anaerobic bacteria also break down organic nutrient but exist in oxygen deficient pond water and soils. Noxious by-products as methane, ammonia and hydrogen sulfide are created by anaerobic decomposition. Foul smelling waters can be assumed to be anoxic or oxygen deficient.
Oxidation is a chemical process that is dependent on oxygen. Oxygen has a positive molecular charge, as an oxygen molecule affixes itself to a particle in the water it then starts to oxidize or break down the molecular bonds which hold the particle together. In addition, the positive molecular charge of the oxygen molecule will create an attraction and pull several small particles together, a process known as coagulation. These heavier, coagulated particles now precipitate, or fall out of suspension. In this process soluble substances like phosphorus and iron become insoluble and unavailable for use by aquatic vegetation. A balanced aquatic ecosystem contains a fairly low population of algae and aquatic weeds as well as other forms of nutrient. Aerobic bacteria feed on the organic nutrients and digest it into non-organic compounds that algae and aquatic plants can not use as readily for food.

Simple water quality tests typically monitor dissolved oxygen, biological oxygen demand, alkalinity, pH, phosphorus, nitrogen, and fecal coliform (in some situations). Dissolved oxygen is described in either parts per million or milligrams per liter. Biological Oxygen Demand is referred to as BOD. Testing can be completed by most water testing laboratories. Understanding these parameters, and how they interact helps with pond management.
Let's sum it up. As a lake ages, levels of nutrient rise due to increases in runoff, organic bottom sediment, or fertilizer. As weeds grow and die and sink to the pond bottom to decompose, one result is a sudden increase in activity and population of aerobic bacteria. Lake depth decreases as biomass at the lake bottom accumulates. Aerobic bacteria uses oxygen to digest organic waste, with primary source of oxygen in the pond coming through surface contact, rainfall and plant photosynthesis.
Due to thermal stratification, the top and bottom layers of water will not mix. Much needed oxygen cannot get to the lake bottom to support aerobic digestion. Oxygen depletion in the lower layers of the lake and may result in nutrient cycling, fish kills and foul odors caused by anaerobic digestion.
Balance is critical to the aquatic ecosystem. Without a balanced environment your pond or lake will suffer. Proper pond construction, including the proper amount of native aquatic plants assists filtering of excessive nutrients. Aeration systems and devices also work to stabilize and balance critical water quality.
Reprinted with permission from Pond Boss Magazine



