When pond owners seek information on water quality from websites, publications, or fishery biologists, alkalinity (or total alkalinity) often is mentioned. This is as it should be, because alkalinity is an important variable in sportfish ponds just as it is for many other water uses. The problem is that alkalinity is a terribly complex and elusive concept, talked about by many and partially understood by few. I will attempt a simple, lucid explanation of the importance of alkalinity in sportfish pond management.
Rain falling on the Earth has been naturally distilled and is relatively pure. Pure water is neutral (pH 7). But, rain water becomes saturated with carbon dioxide while falling through the air. Carbon dioxide is acidic, and in theory, rain water has a pH of about 5.6. Of course, rain water often contains acidic substances such as sulfuric acid from air pollution. This lowers pH - sometimes rain water has a pH below 5. Rain water dissolves many different elements from minerals in soils and other geological formations. The most abundant substances in natural waters are bicarbonate, carbonate, chloride, sulfate, calcium, magnesium, potassium, and sodium. Alkalinity is not in the list of major dissolved substances in water because it is not an actual dissolved substance—it is an index. Alkalinity derives from bicarbonate, carbonate, and a few other basic substances. It is defined as the calcium carbonate (CaC03) equivalent in parts per million (ppm) of the concentration of bases in water, and it is an index of the capacity of water to neutralize acid. It's nature's buffer, or Rolaids, if you will.
Waters of lower alkalinity tend to have lower pH than do waters of greater alkalinity. However, the pH of natural water of a given alkalinity is controlled mainly by its carbon dioxide concentration. A chemical equilibrium exists among carbon dioxide, bicarbonate, carbonate, and pH. Removing carbon dioxide causes pH to increase while introducing carbon dioxide results in a decline in pH. The main factor causing increases and decreases in the carbon dioxide concentration in pond water is photosynthesis. In the daytime, aquatic plants remove carbon dioxide from water faster than it can be replaced by the combined respiration of all pond organisms. Plants cease using carbon dioxide at night, but respiration by pond organisms continues and regenerates carbon dioxide.
The daily fluctuation in pH between the afternoon maximum and the early morning minimum obviously is greater when phytoplankton (or other aquatic plants) are abundant. But, at the same rate of photosynthesis, the daily fluctuation in pH is greater in a pond of low alkalinity than in a pond of higher alkalinity.
In fertile sportfish ponds, phytoplankton often depletes carbon dioxide, causing pH to rise above 8.3. Photosynthesis continues because many species of phytoplankton and other aquatic plants can use bicarbonate as a source of carbon. Carbon availability for plants is greater in water of higher alkalinity than in those of lower alkalinity. In obtaining carbon dioxide from bicarbonate, plants transform two bicarbonate ions into one molecule of carbon dioxide and one carbonate ion. The carbon dioxide is used in photosynthesis, but the carbonate is excreted into the water. The carbonate ion reacts with water, increasing pH. As a result, photosynthesis can cause pH to rise above 8.3—sometimes pH may reach 9 or 9.5 in surface waters of ponds in the afternoon. However, the rise in pH (at the same photosynthetic rate) will be greater at lower than at higher alkalinity.
Water comes in contact with bottom sediment, and bicarbonate and other bases neutralize soil acidity. Ponds with low alkalinity water have acidic bottom soil, and acidity in soil favors rapid removal of phosphate applied in fertilizer. Acidic bottom soils also are not ideal for production of benthic animals that serve as food for pond fish. Microbial recycling of nutrients from sediment organic matter also is diminished by low pH.
Ammonium compounds or urea that converts to ammonium in pond water are applied in fertilizer. Oxidation of ammonium to nitrate by nitrifying bacteria creates acidity that neutralizes alkalinity and lessens pH in ponds with low alkalinity. Another reason that adequate alkalinity concentration is important in ponds is to neutralize acidity from nitrification and other sources.
In water of especially high alkalinity, the pH often is continuously above 8 and there is abundant calcium in the water. These conditions favor the precipitation of phosphate applied in fertilizer.
In summary, alkalinity has several major effects on processes that culminate in sportfish production in ponds as follows:
- determines the background pH—the pH that would occur without additions of fertilizers to promote phytoplankton growth
- the ability of water to resist pH change (buffering capacity) increases with greater alkalinity
- the availability of carbon for photosynthesis increases with increasing alkalinity
- acid conditions in bottom soils of ponds with low alkalinity inhibit the growth of food organisms for fish and retard microbial recycling of nutrients
- factors associated with especially high alkalinity also lessen the availability of phosphate applied in fertilizer
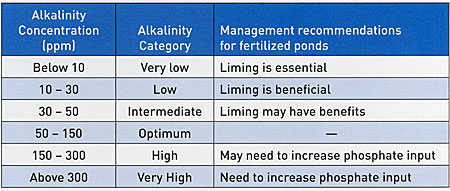
Categories of alkalinity are listed in Table 1. The concentration range for each category is based on the author's experience, and another water quality specialist might offer slightly different categories and concentration ranges. Agricultural limestone should be applied to increase low alkalinity, and a good response to liming usually is realized up to an alkalinity concentration of 30 to 50 ppm in fertilized ponds. Agricultural limestone usually will not dissolve in water with alkalinities above 50 to 60 ppm.
It is significant to note that most pond fertilization research was done in waters with alkalinities of 20 to 60 mg/L. In water with high alkalinity, it may be necessary to increase the phosphate fertilizer input by as much as 50% above the rate used in lower alkalinity water, and in water of very high alkalinity, an even greater increase in phosphate usually will be necessary. It often will be more effective to increase the frequency of fertilizer applications than to use a higher phosphate input per fertilizer application.
There is no effective way of lessening the alkalinity of pond waters. There are references that ammonium from ammonium sulfate fertilizer added to ponds of high alkalinity will be oxidized to nitrate by nitrifying bacteria, creating acidity to lessen alkalinity and pH. Unfortunately, this procedure carries the risk of ammonia toxicity to fish.
Alkalinity typically is needed in areas where soils are highly leached and acidic. Because low alkalinity is a result of a deficiency of limestone and other bases in soils and other geological formation, liming will not cause a permanent increase in alkalinity of pond waters. Liming usually must be repeated at 2 to 5 year intervals. In high alkalinity water, it is necessary to increase the quantity and frequency of phosphate input to establish and maintain a satisfactory phytoplankton bloom. The use of liquid fertilizer or rapidly soluble granular fertilizer is recommended in all ponds, but use of such fertilizers is especially important for ponds with high alkalinity water.
Now that you are convinced of the importance of alkalinity, you may wonder how and how often to measure it. Inexpensive kits for measuring alkalinity can be purchased from sources such as HACH chemical company. Alkalinity does not usually change rapidly. If your pond has above 50 ppm alkalinity, further measurement is unnecessary. When ponds have a low alkalinity, they should be limed, and alkalinity measured again in 4 to 6 weeks to assure that enough liming material was applied. Afterwards it usually is adequate to measure alkalinity at 6- to 12- month intervals so that liming may be repeated when necessary.
Dr. Claude Boyd is a professor in the School of Fisheries, Aquaculture and Aquatic Sciences, Auburn University, Auburn, Alabama 36849. His work with water quality is internationally renowned. His most recent book, Handbook for Aquaculture Water Quality, is a must for anyone interested in learning about water chemistry and how it relates to your pond. It's technical, thorough, but easy to read and understand. Buy it at www.pondboss.com in the online store.
Reprinted with permission from Pond Boss Magazine



