
As biologists, we tend to collect natural facts, file them in our heady little brains, and then use that information during our daily treks into the Held. That's a good thing, except we don't often share some of those most important facts. Here are some of those boring facts, which impact every body of water on the planet...especially yours.
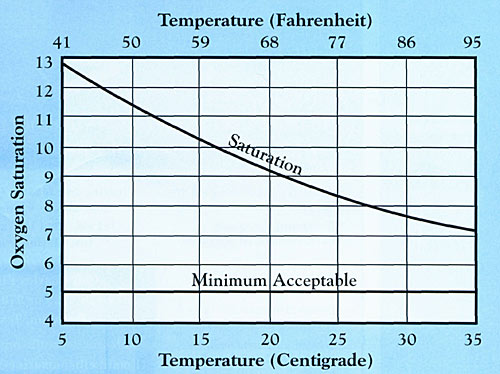
Water is an amazing substance. Its temperature affects its density. Cooler water is heavier than warm water, to a point. Water is most dense at 39 degrees F. Water's affinity for oxygen rises as its temperature drops. What about oxygen saturation? Take a look at the chart below.
Water at 50 degrees holds approximately 11.5 parts per million oxygen. Water at 86 degrees holds slightly less than 8 parts per million. Most fish need a minimum of 3 ppm to survive. So, how do oxygen depletions occur? There are many ways, especially when water is warmest, during summer months. Plants use oxygen, as do fish. During daily dark hours, plants take up oxygen and give off carbon dioxide. Fish breathe, taking up oxygen. With big biomass and hot water, sometimes a pond can't keep up with the demand. The result is oxygen depletion, almost always at the break of day, at the end of the darkest hours of night. At the other end of the spectrum. I'll always remember one pond we checked, early in my aquaculture career. At 4 p.m. oxygen levels were 13 ppm. It was August and water temperatures in the Texas pond were pushing into the low 90's. I knew saturation was less than 8 ppm, so how in the world could my test kit read 13 ppm? Supersaturation. How could that be? Simple. Those same plants which can contribute to oxygen depletion can also be the culprits of excessively high oxygen levels. Plants, during sunlight hours, take up carbon dioxide, use it to create food for growth, and give off oxygen. Big amounts of plants can actually produce more oxygen than water can physically hold. While the water acts as a sponge, soaking up large amounts of oxygen, the liquid also works to release as much of it as possible, to reach its equilibrium, saturation. It simply can't release excessive oxygen into the atmosphere as fast as aquatic plants photosynthesize it.
So next time you stroll past your pretty pond, stand a moment and admire all the fascinating things about the wet stuff, even if you can't see what's going on in it.
Reprinted with permission from Pond Boss Magazine



