Editor's Note: This is a strong science-based article, not easy to understand by many of our readers. Even if you don't understand the terminology, read past it and you'll see the gist and heart of the story.

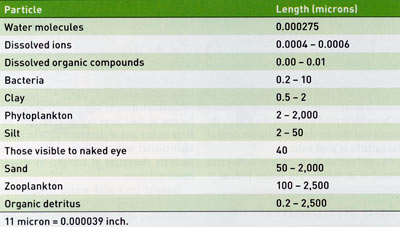
The human eye can see particles that are around 40 microns (0.0016 inch) in size or larger. Many of the particles in pond water are much smaller than visible to humans. Water molecules, dissolved ions, dissolved organic compounds, bacteria, clay particles, smaller silt particles, and smaller phytoplankton organisms in pond water are not individually visible (Table 1). It is interesting to consider the sizes and abundances of things present but not seen in pond water.
The words soluble or dissolved and suspended are used rather carelessly in water quality writings and conversations. These size-related terms have specific meanings. A particle, by definition, is the smallest indivisible portion of matter. Physicists have discovered particles smaller than atoms, but in water quality the atom usually is the smallest particle of concern. Chemists usually consider particles less than 0.5-1.0 microns (about one-hundred-thousandths to two-hundred-thousandths of an inch) to be dissolved, but in water quality the analytical distinction of dissolved and suspended substances is made by passing water through a filter with 2-micron apertures. Particles passing through the filter are dissolved; the ones retained on the filter are suspended. All dissolved and suspended matter is particulate, but the adjective particulate is frequently used in water quality to denote that matter is suspended rather than dissolved.
Let us now turn to considering the size and abundance of water molecules themselves. According to Avogadro's Constant, a gram molecular weight (or mole) of a chemical compound or an atomic weight of an element contains 6.02 ' 1023 molecules or atoms, respectively. The value 6.02 ' 1023 often is called Avogadro's number, and it also can be written as 602,000,000,000,000,000,000,000 or 602 sextillion individual atoms or molecules.
Water molecules are very small, having radii of about 0.275 nanometers which is 0.000275 micron or about one hundred millionth of an inch. Water (H20) has a molecular weight of 18 grams (g), and 1 liter (L) of water weighs 1,000 g. One molecular weight of a substance in 1 L of water often is referred to as a mole. Thus, 1 L of water contains 55.6 moles of water (1,000 g water/L J8 g water/mole). Multiplying by Avogadro's number we find that 55.6 moles of water contains 3.34 ' 1025 molecules (33,400,000,000,000,000, 000,000,000 molecules).
Inorganic ions such as nitrate, ammonium, phosphate, calcium, etc., are slightly larger than water molecules having radii of about 0.0004-0.0006 micron. The largest organic molecules in natural waters are humic substances with radii of 0.001-0.01 micron.
A concentration of 0.05 mg/L of soluble inorganic phosphorus in pond water seems like a very small amount. But, is it a very small number of phosphate ions? Phosphorus in soluble inorganic phosphorus has an atomic weight of 31 g. It follows that 0.05 mg (0.00005 g) of phosphorus represents 1.61 ' 10-6 of an atomic weight of this element (0.00005 g phosphorus i 31 g phosphorus atoms per atomic weight). One atomic weight of phosphorus contains Avogadro's number of phosphorus atoms. Multiplying 1.61 ' 10-6 moles phosphorus/L by Avogadro's number reveals that 1 L of water containing 0.05 mg/L of soluble inorganic phosphorus has 9.7 ' 1017 (940,000,000,000,000,000) phosphorus atoms (or phosphate ions) - a very huge number.
Planktonic algae in a pond must absorb phosphorus from among 3.34 ' 1025 water molecules—there are 3.4 million water molecules [(3.34 ' 1025 water molecules) 3 (9.7 1017 phosphorus atoms)] for each dissolved inorganic phosphate ion. Amazingly, planktonic algae are able to absorb phosphorus from among this vast number of water molecules. But, absorption of phosphorus cannot be by simple diffusion from high to low concentration, because the concentration of phosphorus in aquatic plants is much higher than it is in the surrounding water. Planktonic algae are small and have a large surface area with respect to their volume to increase contact with the water, but the movement of phosphorus into algae still depends upon an active, energy consuming process that can move phosphorus from water contacting the outside surface of the algal cells into the algae.
In addition to dissolved substances, water contains suspended matter consisting of soil particles (mainly fine silt and clay), bacteria, phytoplankton, zooplankton and organic detritus. Smaller soil particles, bacteria, and some of the plankton are not visible to the naked eye (Table 1). Single particles greater than 40 micron are visible, although not in detail. Elevated concentrations of dissolved colored compounds such as humic substances, small clay particles, and small phytoplankters and zooplankters may not be visible as individual particles, but they impart color to water because they scatter and reflect a portion of the light entering a water body. Phytoplankton blooms usually color water some shade of green, while humic substances tint water with a black hue or combine with iron to create a shade of yellow. Water with suspended soil particles appears the color of the soil. The true color of water is the color caused by dissolved particles. Color caused by suspended particles is called apparent color, because it can be removed by filtration. The color we perceive in pond water is a mixture of both true and apparent color. Bacteria usually do not create enough turbidity to be visible in pond water, and this is likely the reason that they are the most misunderstood of the particles in pond water.

A small particle has a very large surface area related to its volume. The volume [volume =(4/3) (3.1416) (cube of radius)] of a single, spherical phytoplankton organism of 50 microns in diameter would be 5.23 ' 10-13 m3, while its surface area [area = (4) (3.1416) (square of radius)] would be 3.14 ' 10-8 m2. In a liter of water, 50,000,000 such organisms (a typical abundance in a fertilized pond) would have a combined volume of 26.2 milliliters and a rather large combined surface area of 1.57 m2 (16.9 ft2).
Small particles of soil are very absorptive because of their large surface area. Also, the large surface area of planktonic algae increases their contact with substances in the water to facilitate absorption of nutrients as already mentioned above.
Seawater has a much greater concentration of major inorganic ions than does freshwater. Nevertheless, light will penetrate as deeply into normal seawater as it will into normal freshwater. Most common ions do not affect water clarity, but large molecules such as those of humic substances interfere with light penetration and impart color to water.
Turbidity is usually beneficial when it results from plankton, because these organisms are the food base for fish. Plankton turbidity also is helpful by limiting visibility into the water to protect fish larvae from predation by large fish. Turbidity also lessens the ability of predaceous birds to see and capture fish from ponds. Finally, reduction in light penetration by plankton turbidity lessens the likelihood of nuisance infestations of aquatic macrophytes (often referred to as aquatic weeds). Of course, too much plankton—particularly phytoplankton—can lead to low concentrations of dissolved oxygen at night and stress or kill fish.
Turbidity from suspended soil particles also limits predation on small culture animals and restricts growth of underwater aquatic weeds, but it restricts light penetration and lessens phytoplankton photosynthesis. Muddy ponds have much lower fish production than ponds with good plankton blooms, and turbidity from soil particles is a negative factor in sportfish pond management.
Dr. Claude Boyd is a retired professor in the School of Fisheries, Aquaculture and Aquatic Sciences, Auburn University, Auburn, Alabama 36849. His work with water quality is internationally renowned. His most recent book, Handbook for Aquaculture Water Quality, is a must for anyone interested in learning about water chemistry and how it relates to your pond. It's technical, thorough, but easy to read and understand. Buy it at www.pondboss.com in the online store.
Reprinted with permission from Pond Boss Magazine



