Over the years, understanding water chemistry has been a difficult chore. Still not sure I understand it well enough. Heck, there's no doubt I still don't understand it enough.
At the same time, I may be a little ahead of some of you, so here goes.
What is dissolved into water influences what grows in it. Some fish can't live in acidic water. Others prefer it. Some plants only grow in acid water and won't grow in water that's too hard. The opposite also holds true. Fish need building blocks, such as calcium, for bones. If it's not in the water, they need a different source. Somewhere in the food chain, if there's calcium in the water, an organism takes advantage of it. Then, something eats that organism, and something eats that organism, and those important minerals, metals, and vitamins move on up into the top of the food chain.
Stability of water chemistry influences what it grows. Rain water is acidic, influenced as it falls by gases in the atmosphere. When the water hits the ground and rolls toward your pond, it grabs stuff as it goes. Some of whatever it grabs either dissolves or is absorbed. Once again, chemistry is influenced. That influence is passed on to living things that want it, or need it.

A few years ago, a loyal Pond Boss subscriber called World Headquarters and I happened to be in the office. He was worried about his pond. Faithfully, he sent water samples to the lab in his home state of North Carolina twice a year. In our conversation he told me his water always stayed around a pH of 5.5. Then, over a period of four years, the pH slowly rose, all the way to 6.8. He saw plant life changing. Spikerush was going that stuff anyway. In its prime, the green carpet-like grass resembling wheat sprouts broke up and floated against the shore by his dock. He also noticed his chain pickerel numbers dropped. He liked that. Being Captain Obvious, I asked if he'd limed the lake. Negative. I asked him if anyone in the watershed limed their hayfields. Nope. I asked him what the surrounding watershed was like. He told me it was a longleaf pine forest. That means sandy soils and lots of tannic acid percolating.
The light bulb went off in his head.
"About five years ago, I made a deal with a guy who brings crews in, rakes the pine straw, bales it and they sell it to landscapers around here. Could that make a difference?" Affirmative.
By cleaning the forest floor, water was less influenced by that, and more by riprap along the dam and native stone in the pond bottom.

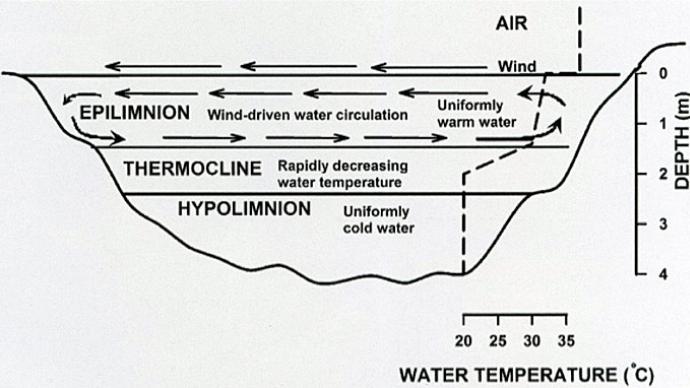
When plants live in your water, as we hope they do, they influence dissolved oxygen. During daylight hours, plants take up carbon dioxide and produce oxygen straight into the water column. The opposite occurs during absence of sunlight.
That process also influences pH, unless your pond is blessed with enough minerals to buffer that change.
When a pond has large standing mats of dense rooted plants, it develops its own internal ecosystem. That ecosystem can biologically influence the water chemistry.
Added organic matter in a pond directly influences local water chemistry. An example of that is the pond bottom's interface with water. At that interface, oxygen demand is so high from bacteria doing what they do, breaking down that organic stuff, that oxygen is consumed faster than it can be added. The pond muck bacteria quickly convert to anaerobic and are much less effective.
The main take home point here is to know the concept. Water chemistry is important. It influences what you want to do. It's also influenced by what you do—or don't do.
Reprinted with permission from Pond Boss Magazine